The EU Health Technology Assessment Regulation (EU HTAR) 201/2282 will change how new pharmaceutical products are evaluated in Europe.
Joint Clinical Assessment (JCA), implemented by EU HTAR, aims to provide faster access to new innovative products to the patients by conducting a combined clinical assessment across the 27 EU member states. This collaborative approach across the member states is anticipated to provide a decision as early as ~100 days after EMA approval of a new innovative product. While it presents an opportunity for faster patient access to new treatments, it also presents challenges for the health technology developers (HTDs) to create dossiers addressing multi-country and stakeholder needs, such as:
- Multiple PICO criteria aligned to individual member states requirements
- Maintaining a 90-day timeline to develop and submit the JCA dossier
- Meeting the 3-month evidence cut-off for literature reviews
Using advanced technologies like Artificial Intelligence (AI) can streamline the process and enable HTDs to meet the requirements of JCA.
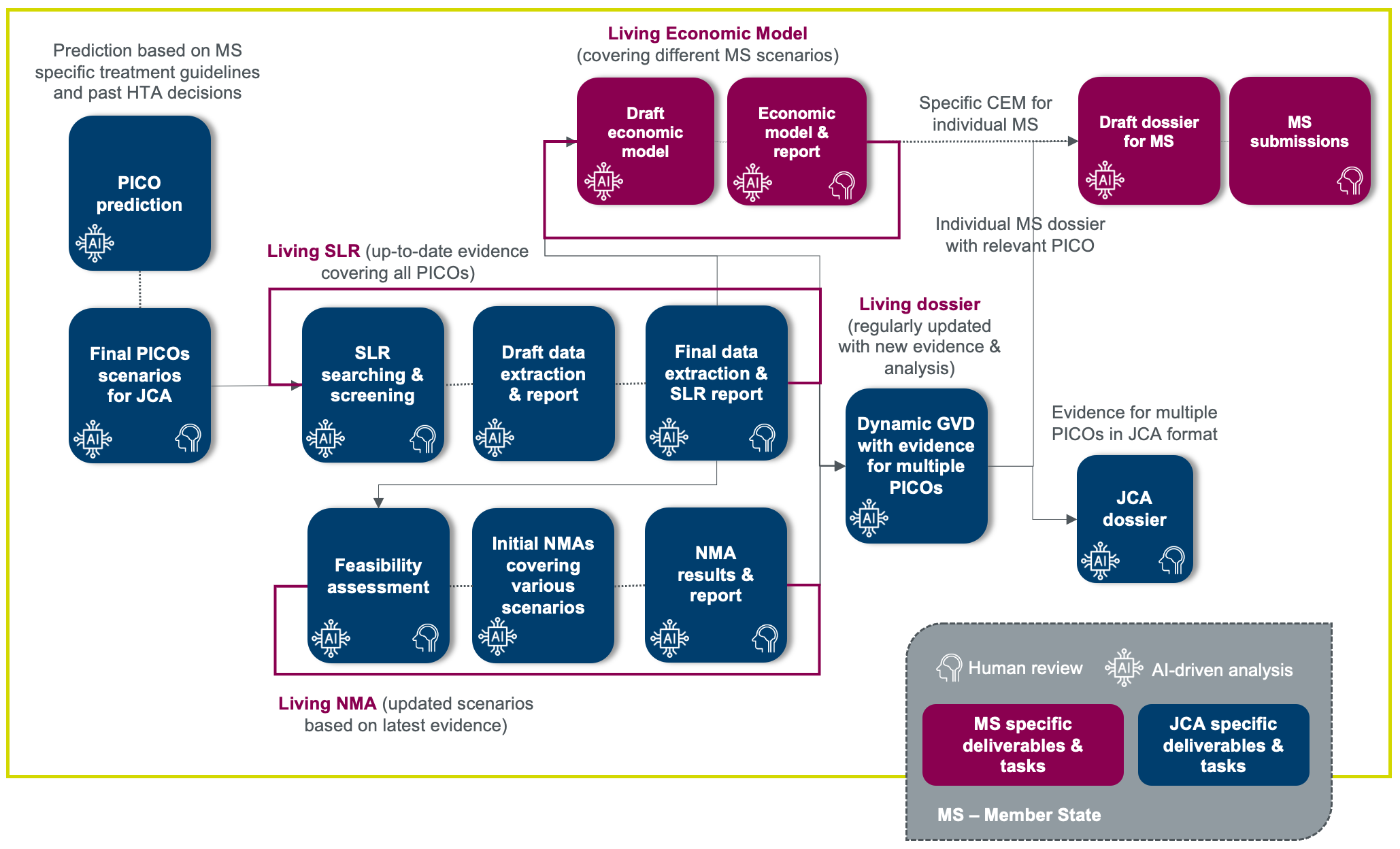
An integrated AI-enabled solution for JCA-related processes
An integrated solution includes AI for predicting PICOs, evidence generation or systematic literature reviews (SLRs), network meta-analysis (NMA), and JCA dossier preparation. While cost-effectiveness modeling (CEM) is not a requirement of the JCA, it is important to evaluate all potential scenarios for member state discussions/submissions after the JCA and develop CEMs in parallel to JCA deliverables.
A key solution component is to ensure the evidence and the analysis is always current and updated to be able to meet the tight timelines, i.e., living.
For strategy development, evidence gathering and analyses for JCA, it is important to estimate the different PICOs that may need to be developed to cover all member state requirements.
Implementation of AI facilitates the synthesis of country-specific guidelines, real-world evidence from clinical practices, and previous HTA decisions to predict the PICOs.
Leveraging AI for conducting SLRs is an integral element in the development of JCA submission dossiers. AI technology offers significant advantages in efficiently handling and synthesizing evidence across various PICO frameworks. Additionally, AI facilitates the seamless integration of the most recent research findings through automated periodic updates.
An AI-driven approach can seamlessly incorporate the most recent data identified through SLRs across various scenarios for feasibility assessment and NMAs. By leveraging AI in this way, the process becomes more dynamic and responsive to newly available information, ensuring that assessments and analyses remain current and allow comparative assessment of outcomes across relevant interventions.
Although CEM is not required for the JCA submission, it plays a crucial role in developing the strategy for product launch and subsequent interactions with individual member states. Therefore, it is essential to maintain and update the CEM to reflect various scenarios based on the most recent evidence and analytical findings. This proactive approach ensures that the model remains relevant, adaptable, and ready for use in diverse national contexts.
Maintain an up-to-date dossier covering all evidence and analyses for different PICOs through the implementation of AI-enabled solutions. AI will also facilitate development of member state specific dossiers with relevant content.
AI-enabled integrated solution
Ethical AI implementation with human oversight and control
Aligned with Parexel’s six principles of an ethical approach to AI solution development, we understand the importance of skilled human oversight when using AI. Our “human-in-the-loop” approach combines AI-driven evidence gathering and analysis with human expert oversight. Humans review, interpret, and contextualize AI outputs, ensuring critical supervision. This collaboration mitigates potential biases and misinterpretations, resulting in comprehensive, efficient, and nuanced analyses that align with human expertise and judgment.
With AI integration in the JCA preparation and submission process, Parexel aims to enable HTDs to navigate regulatory challenges more efficiently, accelerate market entry for innovative pharmaceuticals, and improve patient access to novel therapies.
Our Access Consulting team is always available for a conversation on AI-enabled solutions to support your JCA preparation and submission process. Please get in touch.





