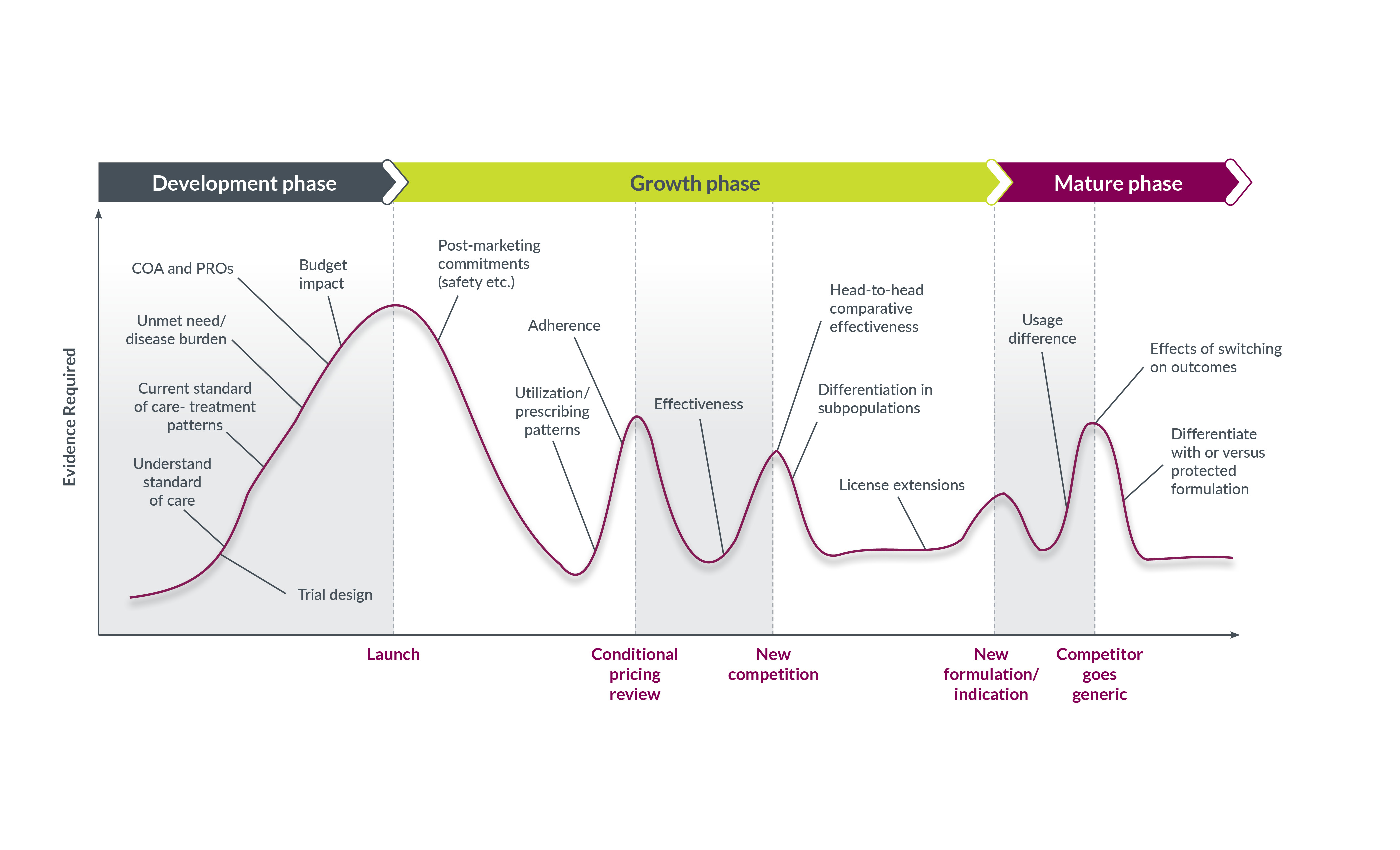
1. Development phase: Validate clinical outcome assessments
Endpoint selection and interpretation remain significant challenges in neurology and psychiatry clinical trials due to subjectivity, self-reporting, heterogeneity, the placebo effect, meaningfulness, sensitivity, comorbidities, and inter-rater variability, among other factors. Establishing the value of a new product requires accurate, sensitive, and validated real-world endpoints, such as clinical outcome assessments (COAs), which, when incorporated into RCTs, can provide holistic, patient- and indication-relevant measurements of disease severity and progression. Regulators have accepted COAs as primary and secondary efficacy endpoints, and payers routinely scrutinize them to assess cost-effectiveness and real-world results.
Choosing optimal COAs—then collecting and analyzing the data with sufficient rigor for regulators and payers—can substantiate an unmet need. For example, schizophrenia’s cognitive symptoms often affect a patient’s ability to work, live independently, and navigate personal relationships. Yet, no schizophrenia therapies are approved to improve patients’ quality of life (QoL) by specifically mitigating cognitive symptoms.
It is critical to validate the construct (the theoretical framework) and content (specific questions, items, or tasks) of COAs. Recently, we helped a sponsor validate caregiver input to the Schizophrenia Cognition Rating Scale (SCoRS), a COA based on interviews with patients, caregivers, or clinicians. We designed a qualitative, non-interventional study to confirm the content and clarity of this scale with paid and professional caregivers.3 Most accurately interpreted the scale’s questions, items, and response options, suggesting that caregivers can assess the impact of schizophrenia-related cognitive issues.
We worked with another sponsor to evaluate the relevance, clarity, and clinical meaningfulness of the Friedrich Ataxia Rating Scale-Activities of Daily Living (FARS-ADL) for patients with spinocerebellar ataxia (SCA), a rare neurodegenerative disorder. SCA presents unique challenges because children are at significant risk of missing crucial milestones in development and are unable to complete self-assessments, even at adolescent age. The goal was to validate the scale’s clarity and ease of use for healthcare providers (HCPs) and its ability to capture clinically meaningful changes. We found that HCPs considered a 1-to-2-point increase in the total FARS-ADL score indicative of clinically meaningful progression and a year or more of stability on any item or the total score as disease stasis.4 The sponsor used FARS-ADL as a secondary endpoint in its pivotal Phase 3 trial and recently filed a new drug application, which the FDA accepted and assigned priority review.
2. Development phase: Identify and enroll target patient populations
Real-world data (RWD) is integral to identifying target patient populations so that new therapies can be tested in a representative sample. If a condition's diagnosis and treatment vary significantly by race, ethnicity, gender, geography, or socioeconomic status, sponsors must understand how to mitigate the risks of under- or overrepresentation in RCTs. Otherwise, HTA agencies and payers may decline to reimburse.
We are working with one sponsor to accelerate representative patient enrollment in a migraine clinical trial. The World Health Organization estimates that non-medical social determinants of health (SDOH)—including access to quality education and healthcare, economic stability, social and community context, and local environment—account for 30 to 55% of health outcomes.5 We characterized three cohorts of patients by diagnosis and treatment and then used claims data to stratify them by education, income, marital status, and SDOH. Our findings confirm that analyzing patient preferences and provider patterns can help identify and recruit a more representative population of migraine patients. Also, the RWD can locate unconventional healthcare providers such as acupuncturists or general practitioners specializing in alternative medicine.
At Parexel, more of our sponsors seek to conduct early-stage trials in patients with Parkinson’s disease (PD) than in healthy volunteers. However, recruiting PD patients into Phase 1 dosing trials is often challenging since they know they will only receive one non-therapeutic dose or a few doses of a treatment. Yet, they may have to stay overnight in a clinic and undergo invasive procedures such as repeated blood draws or a lumbar puncture. We used RWD from a comprehensive U.S. claims data source to target movement disorder experts treating PD patients. Then, to increase enrollment, we targeted community-based sites near large communities of retired people and enrolled them into our network. We will boost recruitment in Phase 1 studies by identifying retired patients who might appreciate the additional care from specialists they might not otherwise be able to afford, and by compensating them for invasive procedures.
3. Development and growth phases: Select reliable, patient-friendly digital technologies
Digital technologies that capture data passively, such as wearable or connected devices, reduce the patient burden, enhancing recruitment and retention. However, those devices must be validated for sensitivity and reliability, patient-friendly, capable of real-time data collection, and able to measure meaningful metrics. This is critical for RCTs and for observational studies.
With neurodegenerative conditions such as Parkinson's disease, patients are busy managing their disabilities. They don't want the additional burden of learning a new user interface or remembering to recharge or synchronize a device.
At Parexel, we have investigated more than 140 connected devices in 300 decentralized trials. For example, we have conducted Duchenne muscular dystrophy (DMD) studies using the endpoint Stride Velocity 95th Centile, which measures walking speed with small devices attached to the ankles using Velcro. The data is transmitted to a centralized database at night while the device recharges. Because wearables record daily movement over a period, the data is unaffected by how motivated or tired a patient may feel during a clinic visit. In 2023, the EMA approved SV95C as a primary endpoint for DMD clinical trials to replace the clinic-based six-minute walk test.6 In the United States, the FDA has approved SV95C as a secondary endpoint.
We recently worked with a national charity organization on digital device and endpoint selection for a longitudinal observational study of a neurodegenerative disorder. The study designers wanted to use an activity tracker and an electroencephalogram (EEG) headset to detect early signs of disease and track progression. Our recommendations included utilizing clinically validated medical-grade devices to safeguard their future results, training patients to ensure compliance and streamline passive data collection, and collecting and retaining as much raw data as possible to leverage any algorithms, aggregation techniques, or statistical analyses that may become available later.
4. Growth phase: Establish safety, effectiveness, and utilization after approval
Sponsors use RWD to conduct post-authorization safety and effectiveness studies, to investigate drug utilization patterns, and to fulfill post-marketing requirements. Real-world post-approval studies demand operational expertise, familiarity with regulatory expectations and industry standards, scientific rigor, and complex data handling and analysis.
Recently, we designed and conducted a mandatory observational cohort study to assess a drug's long-term utilization and safety in a chronic neurological disorder. We used our RWD platform to harmonize and aggregate 100 datasets from three sources. The regulatory agency required annual reports for eight years, so we set up efficient and repeatable data processing procedures. We improved the data quality by cleaning data and resolving discrepancies and conflicts between the sources with a technology-based query management solution.
5. Growth and mature phases: Quantify economic value and differentiate
RWE can differentiate products by collecting data that’s typically unavailable in RCTs, such as medication adherence for a novel drug formulation or dosing regimen. In an RCT, the protocol-specified treatment schedule is strictly enforced, but outside of a trial, patients may not take a drug as directed, adversely affecting outcomes. A real-world study can capture such differences.
Likewise, RWD can quantify the cost of switching from one therapy to another. For example, when an MS patient changes therapies, their utilization of brain MRI scans, medical office, nurse, and emergency room visits, and hospitalizations often increases. A product with durable efficacy and superior tolerability could be more cost-effective than its competitors, but switching costs are not well-characterized. We are helping the sponsor of an MS compound gather and analyze data from an open claims repository and electronic health records (EHRs) to identify the patterns and costs of switching and differentiate their product in a crowded market.
Quantifying the value of a novel treatment requires a sound, disease-specific economic model that demonstrates a disease’s trajectory and impact on the patient’s QoL and productivity in metrics that resonate with payers. However, many economic models are incomplete or outdated.
At Parexel, we continually analyze disease-specific models to ensure they reflect scientific, technical, and clinical advances. For example, we recently analyzed 163 MS models to analyze their development, limitations, and structures and found that less than 10% include magnetic resonance imaging (MRI) data.7 Yet technical advances in MRI—such as quantifying T2 and gadolinium-enhancing lesions and brain volume loss—may have predictive value for long-term disease activity and progression and enable more accurate economic modeling.8 Further, the "gold standard" MS model does not consider fatigue, work productivity, or health resource utilization during relapse and remission cycles. We concluded that there is an urgent need and opportunity for more sophisticated modeling that captures the complexity of MS and can provide better insights into its economic implications.
RWE is vital to sustaining commercial viability
Drug developers should assume that RWE will be an essential component of the value story throughout a neuroscience therapy’s lifecycle. Regulators, HTA agencies, payers, and prescribers all want an evidence-based profile of a new product's benefits and risks before agreeing to recommend, pay for, or prescribe it.
Planning for integrated evidence generation is the surest route to success because it accounts for varying stakeholder needs, maximizes the impact of RWE, reduces redundancies, and ensures that suitable technology is selected for data collection. Generating RWE is a long-term play: work that starts early can determine the success of a launch many years later.
With thanks to the contributors to this article:
Stacy Charlerie – Director, RWD Strategy, Real World Research
Karina D’Angelo – Director, RWD Strategy, Real World Research
Eliza Galvez – Senior Director, Integrated Solutions Strategy
Katja Hakkarainen – Vice President, Head of Epidemiology, Real World Research
Nathan Noakes – Director, Sensor Solutions, Real World Research
Jaime Roberts – Associate Director, Feasibility and Strategy
Jaime Smith – Senior Director, Head of RWD Strategy, Real World Research
Contributing Expert