Harness the power of whole-study solutions
We design and deliver studies that ensure patient safety, data integrity, regulatory compliance, and scientific rigor. Our integrated strategies address these priorities and more, allowing us to reduce burdens, mitigate risk, and operate efficiently within timelines and budgets.
Move your medicine forward with patient-focused research
Data shows that patient-informed protocols increase a drug’s likelihood of launching. That’s one of the reasons we design trials that accommodate patients’ needs and preferences. And by removing barriers to participation, we accelerate study enrollment, boost adherence, and construct stronger value stories.
(Teri Karcher): Study start up has become much more challenging in recent years. There's been an increase in protocol complexity. We have a lot more third party vendor data, and we're seeing at an industry level additional challenges at our investigative sites. And so there's a lot of different and more nuanced approaches that we need to take in order to meet these needs.
Protocol burden is an essential consideration for both our patients and our sites. So, what we do is we provide our expertise into the protocol development early on to make sure that they're not more burdensome than is necessary to meet the scientific needs of our studies.
(Stacy Hurt): We have our internal patient community which are employees at Parexel, who use their lived patient caregiver experience to inform our protocols, we have a patient advisory council of global diverse perspectives. We have relationships with patient advocacy groups all over the world. In addition, we just launched our protocol needs assessment, which shows that when you fulfill patient’s needs as people first, they’re going to be more satisfied with their patient experience in a clinical trial. So it’s really multifaceted approach that we use, utilizing a lot of diverse perspectives to give us a really holistic view of protocol burden and help us solution these for our sponsors.
(Stacy Hurt): A strong patient insights package is really critical to improving the protocol because you have to understand that population that you intend to treat at the very beginning and truly understand what their challenges are, what their needs are, their motivations for participating in a clinical trial, and if you can capture that right at the beginning, then that can inform you all the way from early phase through drug development through late phase all the way through the end of the trial where we commit to providing late patient summaries so patients actually understand the results of the clinical trial journey.
(Teri Karcher): And it's critical to select the right sites for a study in order to recruit the desired patient population. With the right study planning and critical path oversight of our delivery execution, we can benefit patients by bringing the trials to them more quickly. And then this ultimately helps bring the drugs to market more rapidly as well.
(Stacy Hurt): That’s how Parexel is different, because we actually keep the patient’s needs at the center and at the forefront of everything that we do.
Our solution experts
Teri Karcher, Ph.D.
President, Global Project Leadership and Launch Excellence
Deb Tatton
President, Global Clinical & Data Operations
Stephen Pyke, DIC
Chief Clinical Data & Digital Officer
Matthew Gordon
Vice President, Real-World Evidence Strategy
Paul Bridges, Ph.D.
President, Consulting
Andreas Lysandropoulos, M.D., Ph.D.
Senior Vice President, Global Therapeutic Area Head, Neuroscience
Sanjay Vyas
President, Safety & Logistics and Country Head of India
Katie Connelly
Senior Vice President, Global Head of Regulatory Operations
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Our solution experts
Teri Karcher, Ph.D.
President, Global Project Leadership and Launch Excellence
Deb Tatton
President, Global Clinical & Data Operations
Stephen Pyke, DIC
Chief Clinical Data & Digital Officer
Matthew Gordon
Vice President, Real-World Evidence Strategy
Paul Bridges, Ph.D.
President, Consulting
Andreas Lysandropoulos, M.D., Ph.D.
Senior Vice President, Global Therapeutic Area Head, Neuroscience
Sanjay Vyas
President, Safety & Logistics and Country Head of India
Katie Connelly
Senior Vice President, Global Head of Regulatory Operations
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Services
- Phase IIb-IV clinical trials
- Real-world evidence
- Patient engagement strategy and enrollment solutions
- Protocol-driven, customized site solution strategy
- Regulatory strategy, submissions, compliance, and outsourcing
- Market access strategy and delivery
- Clinical development technology optimization
- Clinical trial supply and logistics
- Patient inclusion
- Medical communications
- Drug safety & pharmacovigilance
Phase IIb-IV clinical trials
Today, as the design and conduct of clinical trials continue to evolve, decision-making needs to be informed by strong expertise in biostatistics, data analysis, and data management. This knowledge is essential to ensure patient safety, data integrity, and operational feasibility as flexible protocols become the norm.
At Parexel, we offer this depth of expertise in supporting protocol design, trial execution, site management, and all the way through to market strategy and commercialization. We take a risk-focused approach, continually assessing the tangible risk of the trial so we can control it, adapt to it, and keep everything running smoothly.
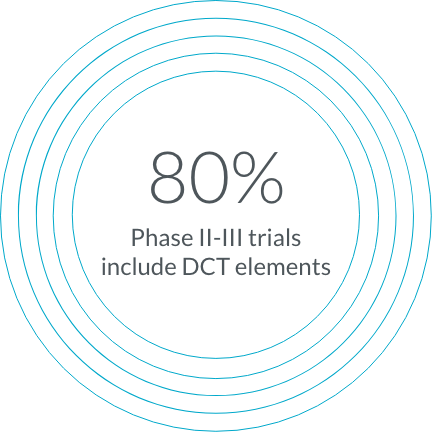
To keep patient needs at the center of your research, we incorporate decentralized trial (DCT) elements into 80% of our Phase II-III trials.
Real-world evidence
Explore the impact of a therapy or disease in a real-world setting, generating data that can support your value proposition without additional burden to the patient. Our real-world evidence (RWE) strategies help reassure payers, regulators, providers, and patients that your clinical results are reflective of what will be evidenced in real-world practice, helping demonstrate value before, during, and after launch.

To access the patients your study needs, we incorporate decentralized trial (DCT) elements into our proposals.
Patient engagement strategy and enrollment solutions
Many clinical trial participants face barriers to participation, from inadequate information about study opportunities to inconvenient times and locations for test visits. As a result, some patient populations have lacked access to possible therapies, and studies have lacked the diversity of participants required to ensure widespread effectiveness.
We focus on the practicalities of patient access and engagement. We recruit widely and track proper population diversity throughout the study. By capturing patient preferences and maintaining appropriate communication with them, we help you recruit and retain the diversity of participants you need.
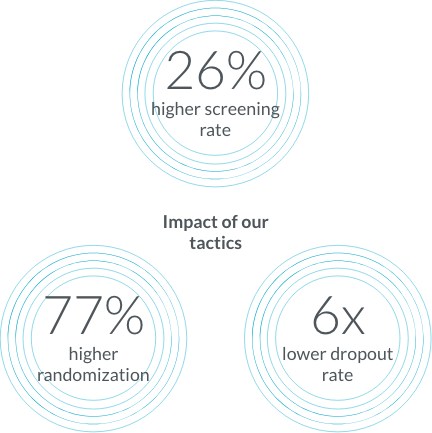
Over the course of 90+ dermatology studies that enrolled 30,000+ patients our patient education and engagement tactics led to better screening rates, optimization, and a lower dropout rate.
Protocol-driven, customized site solution strategy
In response to the increasing complexity of trial protocols and the pressures faced by sites, we accelerate study start-up by helping you find the right sites, patients, and partnerships. Our multidisciplinary Launch Excellence Office aims to meet the specific needs of your study, offering study planning, critical-path oversight, and delivery execution as key elements. We also engage in partnerships with groups offering a broad range of perspectives, so we can design protocol and site-selection strategies with the biggest possible impact in mind.

Our Site Alliance Network gives you access to more sites, diverse populations, and investigators so you can be confident in your site selection strategy.
Regulatory strategy, submissions, compliance, and outsourcing
At this pivotal point in the development process, take control of your regulatory pathway and reduce compliance risks to simplify and accelerate your treatment’s journey to patients.
Our global team of former regulators helped set the standards you’re striving to meet. We’ll help you optimize your regulatory pathway to maximize the value of your product.
Our former FDA, EMA and NMPA regulators, supported by 1,300+ colleagues in local regulatory teams help you manage global complexity in changing regulatory environments.
Market access strategy and delivery
Even with a flawlessly executed clinical trial and a strong regulatory strategy, there’s no guarantee of a successful product launch. Without robust commercialization planning, patient access and return on investment (ROI) may be limited.
We’ll help with that, by delivering a product market access strategy that merges clinical and development plans with regulatory and health economic strategies.

Our market access team has the experience and expertise to help you optimize ROI and patient access, having completed 150+ market access strategy projects in the past 10 years.
Clinical development technology optimization
To make the most of your valuable data, we design fit-for-purpose technology solutions — suites that capitalize on our own mature systems along with best-in-class technology from industry-leading vendors. Each solution is engineered and implemented by experts to address the specific needs of your trial and team.
Our solutions go beyond data collection. We learn about the decisions your team will face, then create ways to curate, visualize, and monitor your data so that you can make those decisions quickly and with confidence.

We won the 2022 Frost & Sullivan Global Customer Value Leadership Award for excellence in planning, operationalizing, and delivering decentralized trials (DCT) worldwide via leading-edge technology.
Clinical trial supply and logistics
Whether conducting a small regional or a complex global trial, you need a robust supply chain network that includes country-specific knowledge and a close connection to local authorities. We provide the knowledge, systems, and connections to achieve end-to-end clinical trial supply chain management. We’ll help you build a supply chain that sails through international pressure points and delivers drugs to patients and trial sites right on schedule.
To ensure that our clients are free to conduct trials around the world, we operate a hub-and-spoke network of depots that seamlessly move drugs and ancillary supplies to investigator sites. Our skilled team of trade compliance professionals will handle customs declarations, apply for permits, and manage interactions with local governments so you can focus on running the best trial for your patients.

We’ve won multiple Asia-Pacific Bioprocessing Excellence Awards, including 2021 Best Clinical Supply Chain, 2020 Best Supply Chain Digitization, and 2019 Most Robust CRO Supply Chain.
Patient inclusion
Clinical trials have often been an exclusive space, rather than an inclusive one, with serious consequences for patients in underrepresented groups. At Parexel, it’s our mission to change that. We’ve gone straight to patients, caregivers, physicians, and community leaders to identify factors that prevent patients from enrolling in clinical trials.
No matter what steps you’ve taken to address equity and inclusion in your trial design process, we’re ready to work with you. We’ll help you design trials that include patients who otherwise might not have been able to participate due to issues such as time, finances, and transportation. By focusing on inclusion, you’ll help more patients while developing drugs and therapies that can work for all.
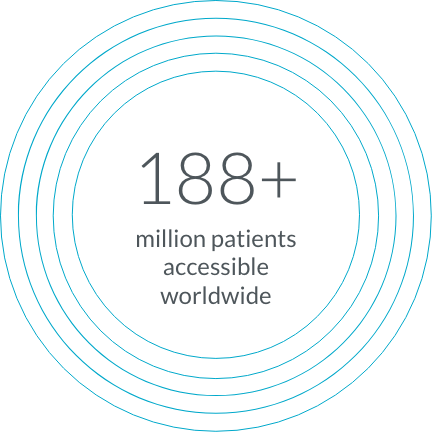
Through TriNetX Live, we’re able to access a network of millions of patients in more than 30 countries, so you can access the diverse populations you need.
Medical communications
Engage the people who matter most. At Parexel, we combine a comprehensive portfolio of medical communications services with expertise in all major therapeutic areas, as well as clinical development, patient engagement, real-world evidence, health economics, market access, regulatory, and more — to communicate vital data to your stakeholders.
Our team consists of Medical Affairs strategists who collaborate with highly skilled PhD, PharmD, and MD writers, seasoned medical editors, meeting logistics experts, and creative, digital, and design specialists. Together, we enhance educational experiences and bring your science to life across every stage of development.

Our writers have deep experience across therapeutic areas, resulting in more relevant, impactful medical communications.
Drug safety & pharmacovigilance
In the rapidly evolving landscape of innovation-driven medicine, ensuring drug safety has become increasingly complex. With vast amounts of data from diverse sources, managing safety information is more challenging than ever. At Parexel, we leverage nearly 40 years of expertise to navigate these complexities, providing comprehensive pharmacovigilance services that prioritize patient safety throughout a product's lifecycle. Our integrated approach combines cutting-edge technology and AI-powered solutions to deliver actionable insights and streamline processes.
With a global team of over 3,000 safety professionals, we support a wide range of therapeutic areas, ensuring regulatory compliance and unwavering commitment to patient health. Explore how our tailored solutions can help you meet the challenges of modern drug safety.
Operational excellence
Constantly evolving how we deliver trials
The purpose of our Operational Excellence and Delivery Office is to continuously and consistently improve the way we run your trials. By assembling our most experienced, cross-functional team members, we create best practices business wide that accelerate timelines, generate compelling evidence, promote innovation — and empower us to deliver With Heart™.
Related Insights
Blog
Accelerated approval: Navigating FDA’s recent guidance and confirmatory trial considerations
Mar 6, 2025
Blog
To build a state-of-the-art oncology site network, we went back to basics
Mar 3, 2025
Blog
Regulatory strategies for ADCs
Feb 28, 2025
Playbook
Streamlining development in the EU: Strategies for smoother CTA submissions
Feb 27, 2025
Playbook
Streamlining success: How integrated evidence planning transforms asset development
Feb 27, 2025
Podcast
Enabling Successful Sites: Episode 5, Part One: Strategies for improving communication between sites, sponsors and CROs
Feb 24, 2025
Blog
SCOPE 2025: Outsourcing in the Era of Agile Biopharma Innovation
Feb 12, 2025
Blog
Navigating the path to conditional approval: avoiding pitfalls in oncology drug applications
Feb 4, 2025
Webinar
Maintain EU-CTR compliance: Guidance on the first substantial modification after slim transition and ongoing strategic information management
Feb 3, 2025
Article
Key strategies to mitigate the risks of asthma drug development
Jan 24, 2025
Blog
Overcoming risks in Phase 3 trials to accelerate time to market
Jan 22, 2025
Blog
Proof of product safety and efficacy: how to move ahead successfully in Phase 2 clinical development
Jan 22, 2025
Blog
Updated European Society of Cardiology guidelines: opportunities and risks for clinical trials
Jan 21, 2025
Webinar
Accelerated Early Phase Decision-Making – Insider Insights to Speed Development
Jan 7, 2025
Webinar
FDA Rare Disease Innovation Hub: Enhancing Collaboration and Speed?
Jan 7, 2025
Blog
Focusing on value drivers to reduce risk in early-stage drug development
Jan 6, 2025
Blog
Biosimilar reference medicinal product (RMP) regulatory requirements: China, US and EU comparison
Dec 20, 2024
Blog
Bridging sponsors and sites: The changing role of the CRA
Dec 17, 2024
Blog
Oral phenylephrine removal from OTC nasal decongestants: next steps for sponsors
Dec 13, 2024
Related Insights
Blog
Accelerated approval: Navigating FDA’s recent guidance and confirmatory trial considerations
Mar 6, 2025
Blog
To build a state-of-the-art oncology site network, we went back to basics
Mar 3, 2025
Blog
Regulatory strategies for ADCs
Feb 28, 2025
Playbook
Streamlining development in the EU: Strategies for smoother CTA submissions
Feb 27, 2025
Playbook
Streamlining success: How integrated evidence planning transforms asset development
Feb 27, 2025
Podcast
Enabling Successful Sites: Episode 5, Part One: Strategies for improving communication between sites, sponsors and CROs
Feb 24, 2025
Blog
SCOPE 2025: Outsourcing in the Era of Agile Biopharma Innovation
Feb 12, 2025
Blog
Navigating the path to conditional approval: avoiding pitfalls in oncology drug applications
Feb 4, 2025
Webinar
Maintain EU-CTR compliance: Guidance on the first substantial modification after slim transition and ongoing strategic information management
Feb 3, 2025
Article
Key strategies to mitigate the risks of asthma drug development
Jan 24, 2025
Blog
Overcoming risks in Phase 3 trials to accelerate time to market
Jan 22, 2025
Blog
Proof of product safety and efficacy: how to move ahead successfully in Phase 2 clinical development
Jan 22, 2025
Blog
Updated European Society of Cardiology guidelines: opportunities and risks for clinical trials
Jan 21, 2025
Webinar
Accelerated Early Phase Decision-Making – Insider Insights to Speed Development
Jan 7, 2025
Webinar
FDA Rare Disease Innovation Hub: Enhancing Collaboration and Speed?
Jan 7, 2025
Blog
Focusing on value drivers to reduce risk in early-stage drug development
Jan 6, 2025
Blog
Biosimilar reference medicinal product (RMP) regulatory requirements: China, US and EU comparison
Dec 20, 2024
Blog
Bridging sponsors and sites: The changing role of the CRA
Dec 17, 2024
Blog
Oral phenylephrine removal from OTC nasal decongestants: next steps for sponsors
Dec 13, 2024






