Where better medicines begin
Early phase decisions are mission critical to the long-term success of your promising innovations. Learn how Parexel’s multi-disciplinary early phase approach can bolster your clinical evidence, accelerate the path to proof of concept and reduce the white space between early and late phase development, so you can deliver high impact therapies to patients sooner.
Early clinical development needs to move fast and deliver the right data to inform critical decisions and build a strong foundation for future success. Rely on Parexel to effectively integrate a breadth of disciplines to navigate complexity and optimize study designs and conduct to accelerate decision-making and determine the best course for your asset.
- Regulatory expertise – Expert guidance on global regulatory strategies and early engagement with regulators before initiating a clinical development program – including advice on the best opportunities for fast track/breakthrough therapy designations.
- Translational medicine – Application of genomic expertise and state-of-the-art methodologies to help clients deliver novel therapies that treat patients based on their unique genomic and biomarker characteristics.
- Clinical pharmacology CoE – Scientific, operational and regulatory expertise in support of study designs, analysis of data and interpretation of study results, strategies for development and registration of drugs in various indications in all major regulatory regions.
- Model-informed drug development – Quantitative science solutions to guide clinical trial design, dose selection, and target population identification.
- Global site network with in-house EPCUs – offers comprehensive capabilities and flexible solutions for studies involving healthy volunteers, niche populations and patients from first in human to proof-of-concept.
- Portfolio due diligence and clinical development strategy – Acceleration of emerging science into promising assets backed by actionable development and commercialization strategies. Clinical development planning and program oversight ensures efficient, connected development from early phase to regulatory submission.
"A multidisciplinary approach is the most cost-effective way to navigate early phase complexity and mitigate risks, and quickly transition to later phase development."
-Oliver Fuhrmann, Executive Vice President, Head of Early Phase Development and Regional Head, Clinical Research Innovations, Europe
Early insights for more productive development
- As a leader in biopharma strategy and clinical development services, we can map out the path to regulatory and commercial success, beginning with asset prioritization strategies, and target product profile (TPP) development through early scientific consulting to clinical development plans.
- Translational medicine encompasses advances from the search for useful biomarkers to biosimulation of disease systems, to use of DNA profiling in clinical trials. At Parexel, we use next-generation analytic tools and technologies to help clients identify diagnostic, prognostic, and predictive biomarkers that can be validated in subsequent development.
Regulatory strategies that make a difference
- Tap into our insider ‘know how” to make the most of early engagement meetings with regulators and minimize the potential for development delays. These consultations are as critical to success as a pre-new drug application or pre-marketing authorization application meeting. Parexel’s regulatory consulting team help clients prepare for these high-impact early advice meetings. Decades of regulatory expertise make Parexel particularly effective when guiding sponsors on how they can best use fast-track/breakthrough therapy designations.
100+ IND/IMPDs and 80+ meetings with the authorities are covered annually by our team of regulatory experts - With a breadth of experience in clinical, nonclinical and CMC, regulatory experts review and assess available data based on their extensive experience with similar development programs to evaluate any potential gaps in data packages for regulators and recommend action to fill each gap, ensuring smooth development progression.
Capabilities and innovation to enable smarter and faster studies
Advanced techniques - We advise clients to use all the available preclinical pharmacokinetic and pharmacodynamic (PK/ PD) data to predict human PK/PD. Using quantitative approaches, our modeling and simulation team helps client optimize the development of promising candidates and eliminate weaker ones earlier in development. In some cases, modeling and simulation can support trial design or fully replace an early clinical study.
Global early phase partner ecosystem with embedded early phase clinical units -- From first-in-human to patient studies, rigorous oversight from experienced staff and global state-of-the-art facilities, supported by standardized procedures provide the right environment to protect patient safety and deliver integrated data. Our early phase site network includes more than 50 sites across five continents.
Deep expertise in the use of adaptive designs and integrated protocols that combine sequential early phase studies into a single protocol to accelerate go/no-go decision making. Parexel’s experience extends beyond design into the operationalization of protocols that are both precise enough to satisfy regulatory requirements and flexible enough to allow the lessons learned in each step to be applied to subsequent cohorts.
As a pioneer in the design and conduct of ethnobridging studies involving Asian populations, we’ve expanded this approach as a cost-effective way to include other sub-populations in early phase studies.
Services
Phase I clinical trials
In Phase I, we test your drug in humans for the first time. Drawing equivalence between two different species, with different enzymes and biology, is technically challenging, but our translational science team takes the animal data you’ve acquired and precisely translates it into human data, resulting in a quick and reliable move to a first-in-human trial.
Human testing is heavily regulated across jurisdictions. Our experience and relationships with major regulatory bodies help determine where and when to start a first-in-human trial and generate the validating data you need. Our network of early-phase partner clinics and our experienced teams in clinical pharmacology and modeling and simulation support you in fast and reliable progress through Phase I.
Site Alliance Network and KOL engagement
Both initial recruitment and retention are increasing problems for clinical trials, resulting in delays, early study closings, and less reliable results. It can be difficult for potential study participants and their physicians to learn about clinical trial opportunities. The complexities of trial start-up impose additional delays. All of this slows the progress of research.
We provide a network of sites and investigators, as well as connections with community and specialist physicians, so patients have a path to studies through their established care delivery systems. Our IRB partnerships speed up approval, and our established relationships with key opinion leaders enable sponsors to identify possible barriers before the start of protocol development.
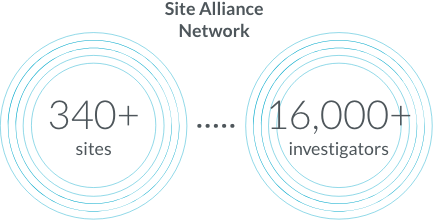
Site Alliance members partner with Parexel to improve research and the patient experience. We continuously look for ways to make study launch activities more efficient.
Regulatory strategy
Regulatory planning and risk mitigation at this early stage of development is a major contributing factor to successful drug development outcomes. Partner with our global team of ex-regulators and industry specialists in the early phases of product development for regulatory intelligence, strategic regulatory advice, agency consulting and engagement, regulatory gap analysis, product development plans, and more.
Our global team of consultants includes former FDA, EMA, and NMPA regulators, so you can advance your trials with confidence.
Market access strategy and delivery
Build the complete evidence package that payers require, from the outset. At Parexel, we’re perfectly positioned with fully integrated regulatory, access, HEOR, RWE, and patient-reported outcomes (PRO) services to collect the right evidence. Starting early is key, and as a result, you benefit from a real-world point of view relevant to reimbursement while developing and adapting your product’s evidence-driven value story throughout the research lifecycle.

Our global market access team is prepared to set your project up for success, right from the beginning.
Biomarker and genomic medicine strategy
Bioanalysis and genomic research are critical to drug development and trial design. When you identify target biomarkers and genetic variants early in the development process, you’ll have a clearer idea of the steps you need to take to turn your new drug into a reality. You’ll be able to select patients for your study with greater accuracy and confidence, ask the right questions when designing your trial, and better articulate the benefits of your drug when it’s time to enter the market.
At Parexel, we offer a comprehensive portfolio of services to help you develop a winning biomarker and genomic medicine strategy.
A recent study found that clinical trials using biomarkers are twice as likely to succeed as those that don’t, making our biomarker strategies all the more vital to your project.
Medical communications
Engage the people who matter most. At Parexel, we combine a comprehensive portfolio of medical communications services with expertise in all major therapeutic areas, as well as clinical development, patient engagement, real-world evidence, health economics, market access, regulatory, and more — to communicate vital data to your stakeholders.
Our team consists of Medical Affairs strategists who collaborate with highly skilled PhD, PharmD, and MD writers, seasoned medical editors, meeting logistics experts, and creative, digital, and design specialists. Together, we enhance educational experiences and bring your science to life across every stage of development.
Our writers have deep experience across therapeutic areas, resulting in more relevant, impactful medical communications.
Operational excellence
Constantly evolving how we deliver trials
The purpose of our Operational Excellence and Delivery Office is to continuously and consistently improve the way we run your trials. By assembling our most experienced, cross-functional team members, we create best practices business wide that accelerate timelines, generate compelling evidence, promote innovation — and empower us to deliver With Heart™.
Related Insights
Blog
Proof of product safety and efficacy: how to move ahead successfully in Phase 2 clinical development
Jan 22, 2025
Blog
Smarter designs, faster: Accelerating decision-making in early-phase development
Dec 10, 2024
Webinar
Accelerated Early Phase Decision-Making – Insider Insights to Speed Development
Jan 7, 2025
Our solution experts
Oliver Fuhrmann, Ph.D.
Executive Vice President, Head of Early Phase Development and Regional Head, Clinical Research Innovations, Europe
Angela Qu, M.D., Ph.D.
Senior Vice President, Translational Medicine, Global Head of Biomarkers & Genomic Medicine
Stanford Jhee
Corporate Vice President, Scientific Affairs
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Matthias Kruse
Vice President, Technical - Regulatory Strategy
Xoli Belgrave
Executive Director, Drug Development Services
Our solution experts
Oliver Fuhrmann, Ph.D.
Executive Vice President, Head of Early Phase Development and Regional Head, Clinical Research Innovations, Europe
Angela Qu, M.D., Ph.D.
Senior Vice President, Translational Medicine, Global Head of Biomarkers & Genomic Medicine
Stanford Jhee
Corporate Vice President, Scientific Affairs
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Matthias Kruse
Vice President, Technical - Regulatory Strategy






