Capitalize on your portfolio’s full potential
We optimize portfolios through strategies that consider every aspect of drug development. Acting on our whole-picture guidance, you can make earlier, faster, more informed decisions about risk and reward — giving each asset its greatest chance for success.
Tap into integrated expert advice
Our team includes experts in clinical, regulatory, and market access strategy. We analyze your assets from multiple vantage points, offering seamless guidance early in development. Through comprehensive and actionable plans, we address development challenges so you can maximize your investments and clinical efforts.
The power of the joint Parexel and Health Advances team is really unparalleled, especially around questions of portfolio optimization early in the journey.
I don't think you'll see a better team that has the depth of expertise across all the functions and dimensions that you really need to think about at the earliest stages.
At Parexel Regulatory, you have ex-regulators that understand I&D submissions, early development decisions, proof of concept. You really have to make those calls on development that will ensure and increase your probability of success to get through the early regulatory milestones.
We have medical directors that have run clinical trials at almost every therapeutic area so they can bring very early insight about getting to the clinic and that translational thinking that you need to do when you're at the earliest stages of development.
So having that strong depth of knowledge that can really engage with our client stakeholders and provide that integrated perspective along all the critical dimensions that you need to balance through those early stages.
Again, I don't think you'll find that anywhere else. I’m proud of what we do at Parexel and Health Advances in terms of how we really partner closely with our clients and make their businesses our own. I see everyone at this company so committed, being part of the client teams, staying focused on what’s right for the patient. I would say, if you’re going to be in this industry, this is where I’d want to be and I’m proud to work here.
I love our focus on patients, right, with heart, kind of that whole culture of doing what’s right by the patient and I think the fact that that’s so ingrained in the culture and all that we do at Parexel is something that’s fantastic.
I love that we make it pretty easy to reach across the different divisions so I’m at Health Advances but I can very quickly engage my colleagues in regulatory consulting, the medical directors are readily accessible, so we really do work pretty nimbly together and I love that, that we all will kind of cross departments to align and work on a tough problem, something that our clients are facing because we’re all aligned on the mission. It’s really that strength of that mission that I find pretty powerful
Our Solutions Experts
Sheela Hegde
Managing Partner & Global Head, Health Advances
Paul Bridges, Ph.D.
President, Consulting
Stephen Pyke, DIC
Chief Clinical Data & Digital Officer
Matthew Gordon
Vice President, Real-World Evidence Strategy
Gwyn Bebb, M.D., BM, BCh, Ph.D.
Senior Vice President, Global Therapeutic Area Head – Oncology
Angela Qu, M.D., Ph.D.
Senior Vice President, Translational Medicine, Global Head of Biomarkers & Genomic Medicine
Teri Karcher, Ph.D.
President, Global Project Leadership and Launch Excellence
Our Solutions Experts
Sheela Hegde
Managing Partner & Global Head, Health Advances
Paul Bridges, Ph.D.
President, Consulting
Stephen Pyke, DIC
Chief Clinical Data & Digital Officer
Matthew Gordon
Vice President, Real-World Evidence Strategy
Gwyn Bebb, M.D., BM, BCh, Ph.D.
Senior Vice President, Global Therapeutic Area Head – Oncology
Angela Qu, M.D., Ph.D.
Senior Vice President, Translational Medicine, Global Head of Biomarkers & Genomic Medicine
Services
Building patient insights into assets, profile, and claims
Patients are central to smarter drug development. We gather and interpret patient input, transforming information into insights that result in more powerful target product profiles (TPPs). When you understand patient needs, you can focus on the assets most likely to find success with target populations as well as health care providers, regulators, and payers.
Our team supports patient-informed portfolio management through rigorous research and our established relationships with a wide range of patient advocacy groups. We engage a diverse mix of people within target populations for a full view of patient challenges and preferences. Because our insights have implications for all stages of compound development, we deliver data early in the process and provide detailed recommendations for putting our findings to use.
Through DataWorks, we have access to millions of diverse patients across the US instantly to help inform your study.
Portfolio optimization
Through industry-leading expertise, we help sponsors pursue the right targets at the right time. Our evidence-based, integrated strategies are backed by deep clinical, regulatory, and commercial knowledge. Drawing on that experience, we can recommend ideal models, map the most efficient regulatory pathways, and forecast your asset’s valuation based on complex market factors. Working as a unified team, our consultants fully equip you to identify and prioritize compounds that demonstrate the strongest potential so you can maximize every investment you make.
Because drug development is so complex, we help you dial in on your most promising products to drive a successful portfolio.
Asset valuation and indication prioritization
Determining the value of an asset and its optimal indications will set the course for product development. We provide analysis, forecasting, and financial modeling so you can assess every opportunity and make the pivotal calls that move your asset ahead.
By analyzing scientific, clinical, and commercial data, our team delivers expert valuations rooted in our nuanced understanding of market movement and evolution. We use this knowledge, along with our proven regulatory intelligence, to prioritize indications. Our recommendations take into account market access considerations as well as an asset’s prospects for approval and the likely length of its regulatory process as determined by our experts, many of whom are former regulators themselves.
Our health economic models provide the information you need to prove your products' value
Early evidence review
Through early evidence consulting, we remove barriers to regulatory approval and market acceptance. Our expert team, which includes former regulators, profoundly understands your asset class, the implications of your data, and what global health authorities will look for in your evidence package.
In reviewing efficacy and toxicology assay and formulation data, we provide insight on every facet of evidence package preparation, including regional differences in evidence acceptance. Through nuanced geographic strategy, we keep development progressing in receptive regions as you generate additional evidence for broader regulatory approvals. In addition to consulting, we can analyze and package your data, submit the resulting drug applications, and consult on subsequent health authority interactions — a full-service approach to this critical process. We also bring our commercial focus to early evidence, evaluating packages for market competitiveness.
Our team of former regulators and HTA professionals help you take control of your regulatory path to reduce risk and accelerate your treatment forward in development.
Model-based drug development
To reduce costs and timelines and mitigate uncertainty, we model and simulate a drug’s likely effects in patients before your clinical trial begins. Modeling can quantitatively justify trial design, dose selection, and mid-trial decisions. It can also support robust regulatory evidence packages.
Because we work with many quality labs, we can recommend the ideal facility for any model-determined test you need to conduct. This partner-agnostic approach ensures that tests are performed by the best-suited vendors — and we can advise on and manage the entire process.
Integrated development strategy and planning
Successful drug development begins with promising science, but you’ll also need to navigate complex regulatory and commercial landscapes. To give your assets every advantage, begin with an integrated development strategy.
Our strategy and tactical plans consider clinical and regulatory challenges as well as commercial positioning. We work closely with you to develop a target product profile to guide our efforts, and our regulatory experts (many of whom are former regulators) can advise on what designs and data packages health authorities will accept. Our clinical experts provide insight on the feasibility of study design, recommending alternatives if needed. We also test a product’s place in the market to understand how it will likely be received by payers, patients, and providers. Using all of this input, we create a comprehensive strategy that can map your path all the way to approval, maximizing your potential for clinical and commercial success.
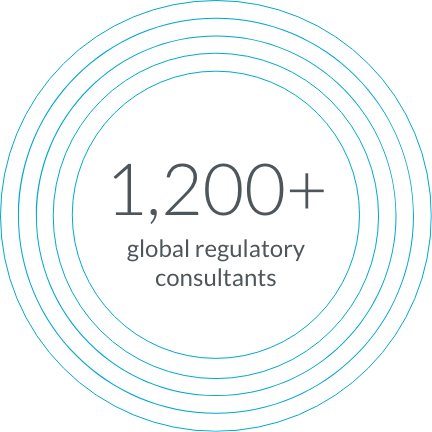
Our global team of consultants includes former FDA, EMA, and NMPA regulators, so you can advance your trials with confidence.
Operational excellence
Constantly evolving how we deliver trials
The purpose of our Operational Excellence and Delivery Office is to continuously and consistently improve the way we run your trials. By assembling our most experienced, cross-functional team members, we create best practices business wide that accelerate timelines, generate compelling evidence, promote innovation — and empower us to deliver With Heart™.
Related insights
Blog
Accelerated approval: Navigating FDA’s recent guidance and confirmatory trial considerations
Mar 6, 2025
Blog
Regulatory strategies for ADCs
Feb 28, 2025
Playbook
Streamlining development in the EU: Strategies for smoother CTA submissions
Feb 27, 2025
Whitepaper
Early and often: reimagining patient community engagement to improve clinical trials feasibility
Feb 27, 2025
Playbook
Streamlining success: How integrated evidence planning transforms asset development
Feb 27, 2025
Podcast
Enabling Successful Sites: Episode 5, Part One: Strategies for improving communication between sites, sponsors and CROs
Feb 24, 2025
Blog
Navigating the path to conditional approval: avoiding pitfalls in oncology drug applications
Feb 4, 2025
Webinar
Maintain EU-CTR compliance: Guidance on the first substantial modification after slim transition and ongoing strategic information management
Feb 3, 2025
Article
Key strategies to mitigate the risks of asthma drug development
Jan 24, 2025
Blog
Overcoming risks in Phase 3 trials to accelerate time to market
Jan 22, 2025
Blog
Proof of product safety and efficacy: how to move ahead successfully in Phase 2 clinical development
Jan 22, 2025
Blog
Updated European Society of Cardiology guidelines: opportunities and risks for clinical trials
Jan 21, 2025
Blog
Navigating the Joint Clinical Assessment (JCA) implementation: An analysis of EU member states readiness, challenges and opportunities for harmonization
Jan 9, 2025
Webinar
Accelerated Early Phase Decision-Making – Insider Insights to Speed Development
Jan 7, 2025
Webinar
FDA Rare Disease Innovation Hub: Enhancing Collaboration and Speed?
Jan 7, 2025
Blog
Focusing on value drivers to reduce risk in early-stage drug development
Jan 6, 2025
Blog
Biosimilar reference medicinal product (RMP) regulatory requirements: China, US and EU comparison
Dec 20, 2024
Blog
Oral phenylephrine removal from OTC nasal decongestants: next steps for sponsors
Dec 13, 2024
Blog
How to maintain EU-CTR compliance for studies after slim transition
Dec 10, 2024
Related insights
Blog
Accelerated approval: Navigating FDA’s recent guidance and confirmatory trial considerations
Mar 6, 2025
Blog
Regulatory strategies for ADCs
Feb 28, 2025
Playbook
Streamlining development in the EU: Strategies for smoother CTA submissions
Feb 27, 2025
Whitepaper
Early and often: reimagining patient community engagement to improve clinical trials feasibility
Feb 27, 2025
Playbook
Streamlining success: How integrated evidence planning transforms asset development
Feb 27, 2025
Podcast
Enabling Successful Sites: Episode 5, Part One: Strategies for improving communication between sites, sponsors and CROs
Feb 24, 2025
Blog
Navigating the path to conditional approval: avoiding pitfalls in oncology drug applications
Feb 4, 2025
Webinar
Maintain EU-CTR compliance: Guidance on the first substantial modification after slim transition and ongoing strategic information management
Feb 3, 2025
Article
Key strategies to mitigate the risks of asthma drug development
Jan 24, 2025
Blog
Overcoming risks in Phase 3 trials to accelerate time to market
Jan 22, 2025
Blog
Proof of product safety and efficacy: how to move ahead successfully in Phase 2 clinical development
Jan 22, 2025
Blog
Updated European Society of Cardiology guidelines: opportunities and risks for clinical trials
Jan 21, 2025
Blog
Navigating the Joint Clinical Assessment (JCA) implementation: An analysis of EU member states readiness, challenges and opportunities for harmonization
Jan 9, 2025
Webinar
Accelerated Early Phase Decision-Making – Insider Insights to Speed Development
Jan 7, 2025
Webinar
FDA Rare Disease Innovation Hub: Enhancing Collaboration and Speed?
Jan 7, 2025
Blog
Focusing on value drivers to reduce risk in early-stage drug development
Jan 6, 2025
Blog
Biosimilar reference medicinal product (RMP) regulatory requirements: China, US and EU comparison
Dec 20, 2024
Blog
Oral phenylephrine removal from OTC nasal decongestants: next steps for sponsors
Dec 13, 2024
Blog
How to maintain EU-CTR compliance for studies after slim transition
Dec 10, 2024






