Operationalize post-trial access with a proven platform approach
As a biopharmaceutical industry, we share an ethical and regulatory responsibility to provide post-trial access to treatment for all patients who participate in a clinical study until the product is commercially available, reimbursed and accessible, or until the patient no longer benefits from the treatment.
Traditionally, post-trial access programs are embedded within individual clinical trials. But when a sponsor has multiple studies ongoing or planned for an asset, this approach can create unnecessary cost and increase the burden for sites, patients and caregivers. This ultimately results in operational inefficiency and complexity – and is a missed opportunity to harness the power of longitudinal data for real-world evidence requirements.
Optimized approach for post-trial access and ongoing evidence generation
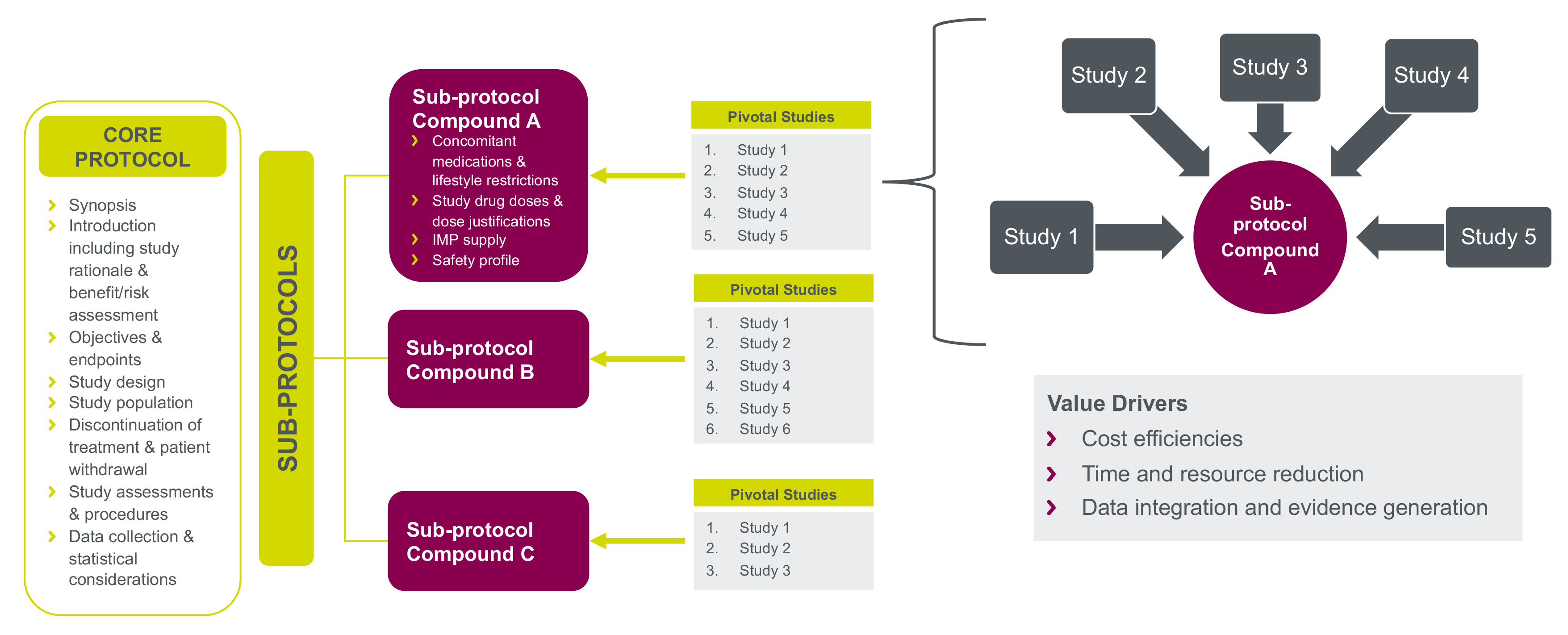
At Parexel, we have developed a platform model to operationalize post-trial access for sponsors running several studies for their product. Our approach is to manage the rollover from multiple pivotal trials into a single core study covering several sub-protocols. This is implemented after the data cut-off, database closure, final analysis or clinical study report for each of the individual clinical studies in scope.
Based on successful implementation with key customers, we have identified value and efficiency creation for all stakeholders.
Patients and caregivers:
- Maintain continuity of treatment
- Fewer procedures and less time spent on study visits through optimized data collection requirements
Sites:
- Reduced burden through minimized data collection requirements
- Improved resource, systems, and tools efficiency through the introduction of a core protocol
Sponsors:
- Adaptable for evidentiary requirements
- Integrated view of patients across the core protocol
- Scalable and rapid deployment for the inclusion of new studies into the post-trial access program
- Operational and process improvements gained, for example, less time spent on repetitive system set-up and cross-system data management
- Long-term budget efficiencies delivered through the management of a post-trial access program through a core study
Partner with Parexel Real World Research to develop and deliver your post-trial access program for:
- Inclusive protocol design, powered by our industry-leading epidemiology team and therapeutic and medical expertise
- Efficient deployment with flexibility, standardization and scalability – including efficiency gains with pre-loaded tools and technology
- A comprehensive solution inclusive of data collection, safety management, regulatory and ethics guidance, site support and supply and logistics services
- Robust governance through a single project leader, with the resources of a global CRO
Our Real World Research team is always available for a conversation about how to create value through your post-trial access program development.
Browse our latest insights
Want to hear from our experts on the latest industry topics? Visit our Insights Center to read, watch, and listen.