Unleash unmatched regulatory insights at this pivotal point in the development process
Early regulatory planning and engagement are fundamental to safeguard your development, avoid pitfalls and set your product up for the best chance of success. Gain insight into how your plans will be evaluated by regulatory agencies, from our team which includes former regulators. With their experience, they interpret guidelines to maximize the potential of your asset development programs.
At this pivotal point in the development process, take control of your regulatory pathway and reduce compliance risks to simplify and accelerate your treatment’s journey to patients. Areas of support include:
- Integrated product development planning: Integrate key components of drug development, including non-clinical, CMC, clinical, medical, regulatory and market access strategies into a cohesive plan. Incorporate these critical areas to minimize delays, improve efficiency and increase alignment among different teams and stakeholders.
- Regulatory agency engagement: Early and frequent engagement with global regulatory authorities is invaluable, and we provide strategic support for these interactions. By formulating an optimal approach to engage with the authorities, we can navigate their expectations and requirements. Leverage our insights and collaborative approach, to confidently and effectively communicate with regulatory agencies.
- Protocol optimization: Consider the data needs of all stakeholders, including patients, regulators and payers. By fine-tuning study protocols, we enhance data quality, to enable more efficient decision-making and facilitate a smoother development process. Our optimized protocols offer meaningful outcomes for patients while meeting the expectations of the regulators and payers.
- First-time quality regulatory submissions: Achieve accurate and complete regulatory documents by prioritizing first-time quality, to increase the chances of obtaining timely approvals. This approach is crucial for maintaining a positive engagement and perception with regulatory authorities.
- Preparation for GxP inspections: With our team of former regulators responsible for conducting global site inspections with the FDA and other regulatory agencies, we understand what’s needed for your facilities meet all necessary compliance requirements. Our proactive approach minimizes the risk of findings or non-compliance issues that could potentially disrupt your operations or cause delays in product approval.
- Outsourcing: To support companies to increase efficiency and resource availability, while maximizing the return on investment for products, we offer outsourced services. Our team handles global registration, functional services (e.g. CMC) and global maintenance activities, freeing up your internal resources to redirect their expertise and attention toward strategic imperatives. This strategic allocation of resources allows you to drive innovation and achieve progress towards your long-term goals whilst ensuring global exposure for your asset, and compliance with local regulations in all markets.
Risk-based quality management with advanced analytics
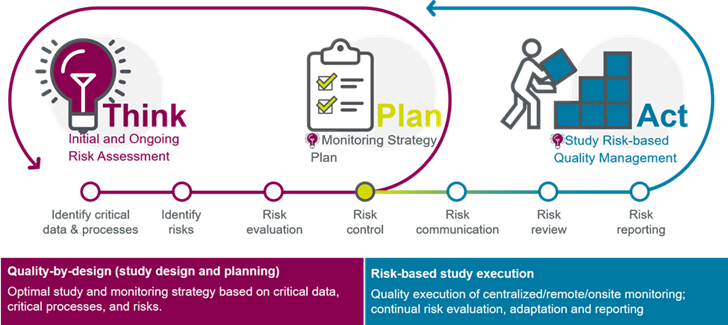
Identify, capture, and manage data quality issues at the earliest stages— before potential risks escalate to liabilities, with Risk-based Quality Management Services (RBQM).This approach forms the basis of a fit-for-purpose monitoring strategy, using study risk assessments, complexity, country requirements, site monitoring, and capabilities as key considerations.
A trusted partner
From the earliest phases of clinical development to marketing approval, and through lifecycle maintenance, Parexel’s 1,300+ regulatory specialists, including former regulators from agencies across the world, provide the knowledge, systems, and technology-enabled processes to accelerate and streamline the medical product development journey. With end-to-end expertise and experience in more than 110 countries, we provide strategic regulatory advice, anticipate and manage risk, and navigate frequently changing regulatory hurdles.
Our Experts
Katie Connelly
Senior Vice President, Global Head of Regulatory Operations
Mwango Kashoki, M.D., M.P.H.
Senior Vice President, Global Head of Regulatory Strategy
Jo Wang
Vice President, Technical
Mark Birse
Technical VP, Parexel Consulting (Compliance)
Anna Zourabian-Scalese
Executive Vice President, Quality Assurance
The Regulatory Navigator
Want to hear from our experts on the latest industry topics? Visit our Insights Center to read, watch, and listen.






