患者主導の医薬品開発のためのパレクセルのソリューション
患者主導の医薬品開発には、患者支援者との30分以上の面談や、ウェブサイト上での意欲をかき立てられるような文章以上のものが必要です。 それは開発の初期段階から始まり、その後のほぼすべての意思決定に影響を与える体系的なアプローチです。 弊社の患者支援部門は、スポンサーが患者さんのインサイトを活用し、複雑化する償還環境に対応し、リスクを軽減し、患者さんに適切な利益をもたらす商業的に実現可能なプロダクトを開発できるよう支援します。
By Katja Hakkarainen, PhD, Vice President & Global Head of Epidemiology, Real World Research
Published on: Jul 8, 2025
10 min

アンメットニーズに対応する製品は、多くの場合、規制当局による審査と償還が迅速化されます。しかし、規制当局、保険者、医療技術評価 (HTA)機関、医療提供者、患者さんの間で、アンメットニーズをどのように定義するかについて合意が得られていません。実際、これらの関係者は、矛盾する価値観、認識、優先順位を持っていることがよくあります。たとえば、国民皆保険制度における保険者は、予算を念頭に置いた医療を多くの人々に提供しなければなりませんが、民間保険者は収益性の高い事業運営を望んでいます。一方、患者さんは常に最善の治療を求めます。
アンメット メディカル ニーズを特定するには、治験依頼者はまず疾患や症状を理解し、文献検索、自然歴研究、専門家へのコンサルティングを通じて、現在と将来の競争状況を評価する必要があります。しかし、それでは全体像がつかめません。アンメットニーズを特定して定量化するには、患者さんの協力が不可欠です。医薬品や治療法の最終的な消費者は患者さんです。彼らは自身の疾患の経過に精通しており、自分たちにとって重要なアウトカムを最もよく理解しています。
何が患者さんにとって重要かの答えや定義は1つではありません。患者さんが最も重要視することは、疾患のステージ、治療方針、利用可能な選択肢、個人的嗜好によって変わるためです。患者さんや患者支援団体 (PAG)にインタビューし、彼らの要望やニーズを聞くだけでは十分ではありません。得られたインサイトは、安全性と有効性を証明するのに十分なデータ収集が必要な臨床試験デザインにおいて、適切かつ測定可能な評価項目に変換されなければなりません。
臨床転帰評価 (COA)は開発の初期段階で活用・検証され、ピボタル試験で使用されることで製品がアンメットニーズを満たしていることを証明できます。たとえば、多くの人がすべてのがん患者にとって、治癒や腫瘍縮小が最優先事項であると考えています。よく耳にするメッセージは、がんと「闘う」や、がんを「取り除く」というものです¹。しかし、進行性がんの患者さんの中には、最新でも非常に毒性が高い治療薬より、効果が低くても副作用の少ない治療薬を好む人もいます。一部の適応症では、これが患者さん中心のアウトカムであり、アンメットニーズを表す場合があります。
病状の異なる患者さんは異なる懸念を抱いている可能性があるため、COAをある病状から次の病状に単純に移行することはできません。また、治療不可能で余命が短い患者さんは、治癒する可能性がある患者さんよりも、症状や副作用のない充実した時間を大切にする場合があります。
治験参加者による紙や電子形式の自己報告です。多くの場合は、症状の重症度を尺度でスコア化したり、特定の事象 (下痢など) の数を経時的に記録したりするよう患者さんに求める質問票になっています。
患者さんを毎日観察する介護者さんやご家族など、患者さん本人や医療専門家以外の人物からの報告です。これも多くの場合、質問票になっています。
患者さんを観察した医療専門家からの報告です。これには臨床的な判断や解釈が含まれる場合があります。
患者さん、または訓練を受けた個人からの報告です。一連の指示に従って実施される標準化されたタスクに基づいています。
何が患者さんにとって重要かの答えや定義は1つではありません。患者さんが最も重要視することは、疾患のステージ、治療方針、利用可能な選択肢、個人的嗜好によって変わるためです。
FDAは患者さん中心の医薬品開発 (PFDD) を優先しており、過去4年間に4種類の指針文書を発行しています²。以下はFDAのPFDDガイドラインから改変したCOAロードマップです。
目的適合性があり、患者中心のエンドポイントを選択するためのロードマップ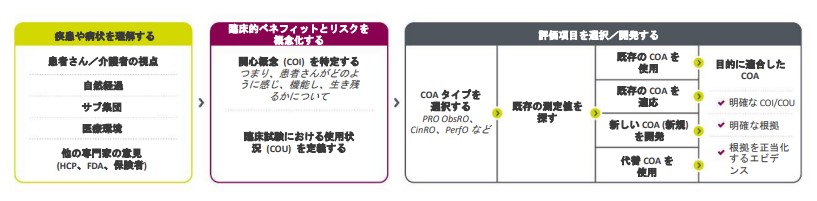
患者さんに関連するCOAツールは通常、投薬中の患者さんのQOLに影響を与える以下の3つの要素を中心にしています。
治験依頼者はCOAを選択する際、侵襲性を見落とすことがよくありますが、これは患者さんの意思決定においては重要な要素です。治験依頼者によっては、薬剤が細胞レベルで効果があるかどうか、または医薬品の適正製造基準 (GMP) に準拠しているかどうかを証明することに集中するあまり、薬剤が患者さんの日常生活にどれほど負担をかけるかを忘れてしまいがちです。たとえば、吸入インスリンは皮下インスリン投与よりも便利で、糖尿病の患者さんにとって画期的な治療法とされました。しかし、さまざまな副作用や医療保険のハードルに加え、既存のペン型注射器と針が大部分の糖尿病患者にとって、ほとんど痛みを伴わないという事実が普及を妨げました³。
治験依頼者は患者さんの視点を取り入れる際に、時間とコストのバランスを考える必要があります。しかし、患者さんにとって重要な転帰に薬剤が及ぼす影響の十分なエビデンスを提出できなければ、規制当局、HTA審査担当者、保険者から異議を申し立てられ、自社製品が失敗に終わる可能性があります。
HTA機関や保険者を説得するために、治験依頼者がQOLに関するエビデンスを提出してプライシングを裏付けなければならない状況がますます増えています。高価な新製品を発売する前に、患者さんのベネフィットに関する説得力あるデータが必要になります。COAの選択は慎重に行うことが重要です。COAは適切に測定された場合、患者さんにとって重要な健康面を反映し、治験薬によって改善され、計画された臨床試験の期間内で試験群間に臨床的に意義のある差異を実証できる必要があります。
パレクセルでは新規または既存のCOAに重要業績評価指標 (KPI) を適用して、目的に適合しているかどうかを判断します。既存のCOAを変更して使用できることもあれば、まったく新しいCOAの開発が必要な場合や、既存のCOAの要素を新しい測定法と組み合わせることが必要な場合もあります。COAに重大な欠陥があると判断した場合は、弊社でよりよいCOAを模索します。
一部のCOAツールには、患者さんが理解しづらいという欠陥があります。たとえば、治療に対する患者さんの「満足度」を測定するためによく使われるPROツールの1つは、「この薬があなたの病状を予防または治療する能力に、どの程度満足していますか、あるいは不満を感じていますか?」という複雑な質問から始まります。患者さんは「非常に不満」から「非常に満足」までの7段階で満足度を評価することになります。これは直感的ではなく、患者さんの意見に基づいて開発されたものではありません。別のPROでは、「薬の副作用が身体活動 (持ち上げる、歩く、ジョギングなど) に支障をきたしていますか?」といった簡単な質問を行います。患者さんは「全く支障がない」から「非常に支障がある」までの5つの回答から選択できます。2つ目のツールの文言は患者さんの意見を参考に作成されました。
重要業績評価指標 (KPI) は企業が患者さんにとって重要なCOAを選択するのに役立ちます。
| 主要なKPI | 患者報告アウトカム (PRO) または観察者報告アウトカム (ObsRO) | 医師報告アウトカム (ClinRO) | パフォーマンスアウトカム (PerfO) |
|---|---|---|---|
| 患者さんとの関連性 | 患者さんと介護者の意見を取り入れて開発されたか? | 患者さんと医師の意見を取り入れて開発されたか? | 患者さん自身で実行できるか? |
| 管理にかかる時間の負担 | 人間の集中力が続く時間よりも長くかかるか? | 限られた医師のトレーニングと再調整が必要か? | テストの実行時間は苦痛を引き起こすか? |
| 解釈可能性 | スコアは患者さんに関連しているか? | スコアは医師と患者さんに関連しているか? | スコアは患者さんとテスト実施者に関連しているか? |
| 読みやすさと複雑さ | 患者さんは質問を理解できるか? | 医師は指示を理解でき るか? |
このタスクは生態学的に有効か? |
| 異文化間の 妥当性 |
その概念はすべての国で有効か? | 臨床試験は各国の医学部で使用されているか? | パフォーマンステストはすべての国で機能するか? |
COAを使用すると、治験依頼者は従来の臨床研究評価項目では測定できなかった患者さんの経験や知覚を測定できます。これほど患者さんを中心にしたものは他にありません。適切に開発、収集、分析されたCOAデータは、規制当局、HTA機関、保険者、医療提供者、患者さんによる意思決定の後押しになります。
臨床転帰評価を使用すると、治験依頼者は従来の臨床研究評価項目では測定できなかった患者さんの経験や知覚を測定できます。これほど患者さんを中心にしたものは他にありません。
Contributing Experts
¹ Let's Stop Talking about "Battling Cancer" (January 2020, Scientific American).
² Patient-Focused Drug Development: Collecting Comprehensive and Representative Input (2020); Patient-Focused Drug Development: Methods to Identify What Is Important to Patients (2022); Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments (2022); Patient-Focused Drug Development: Incorporating Clinical Outcome Assessments Into Endpoints for Regulatory Decision-Making (2023).
³ Commentary: Why Was Inhaled Insulin a Failure in the Market (August 2016, Diabetes Spectrum).
患者主導の医薬品開発には、患者支援者との30分以上の面談や、ウェブサイト上での意欲をかき立てられるような文章以上のものが必要です。 それは開発の初期段階から始まり、その後のほぼすべての意思決定に影響を与える体系的なアプローチです。 弊社の患者支援部門は、スポンサーが患者さんのインサイトを活用し、複雑化する償還環境に対応し、リスクを軽減し、患者さんに適切な利益をもたらす商業的に実現可能なプロダクトを開発できるよう支援します。